10 June 2021
What Are The Technical Requirement Of Fuels?

What are fuels?
For most, fuel is a synonym of petrol and diesel, and is often associated with fossil fuels, pollution and CO2 emissions. If we delve into the dictionary, fuel is defined as “a substance used to provide heat”. At Coryton, we believe there is so much more to fuel than that.
Fuels are not just heat producers
Firstly, fuel must be seen as an energy carrier. There are multiple ways to store energy either as potential energy, kinetic energy, electrochemical energy or as a pure chemical compound. In the case of liquid fuels, the energy is released through a chemical transformation.
In addition to energy content, an energy carrier should:
- Keep your system in good condition. It should not damage or deteriorate your system
- Minimise pollutant emissions
- Be safe to use
- Bring value from a business point of view
- Comply with legislation
- Ideally be part of a recyclable/sustainable supply chain, minimising its life-cycle GHG emissions
Depending on the application (passenger cars, long haul, aviation, marine, etc.), the importance of each of the above points will differ (Fig 1). All these considerations are constantly evolving due to external factors such as economics, politics, technology developments and public opinion.
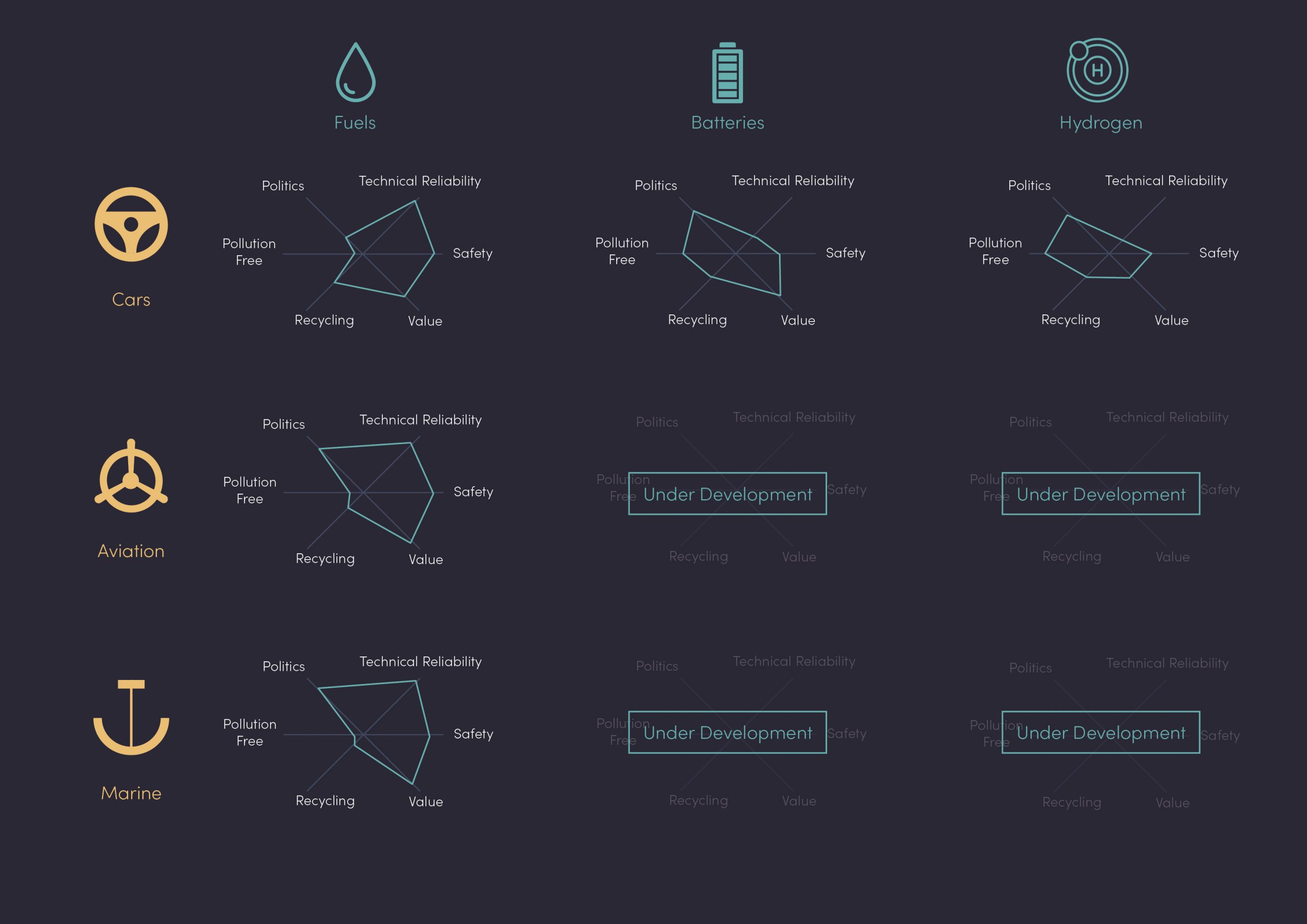
In figure 1, you’ll notice the over-simplification as under names such as “fuels” or “batteries”, there are large families of technology that sit under these which are constantly under development. For example, safety concerns are different for an AA alkaline battery and an NCM-811 car battery, mainly due to the underlying cathode chemistry. The same applies to “fuels” depending on the type of fuel and, more importantly, on its origin (fossil or renewable/sustainable).
Beyond the individual views on each transportation segment, it’s important to understand the energy system is broader than just transportation; it also includes industries such as basic materials (steel or paper production), general manufacturing (car, general appliances, etc.) and even communication and entertainment (email and streaming servers are new sectors that have quickly become major energy consumers).
Another often-hidden crucial point is that all these segments are linked together, so that it is extremely difficult to suppress, or even to diminish, their contribution to the big picture because of refinery processes.
Why all fuels are interdependent
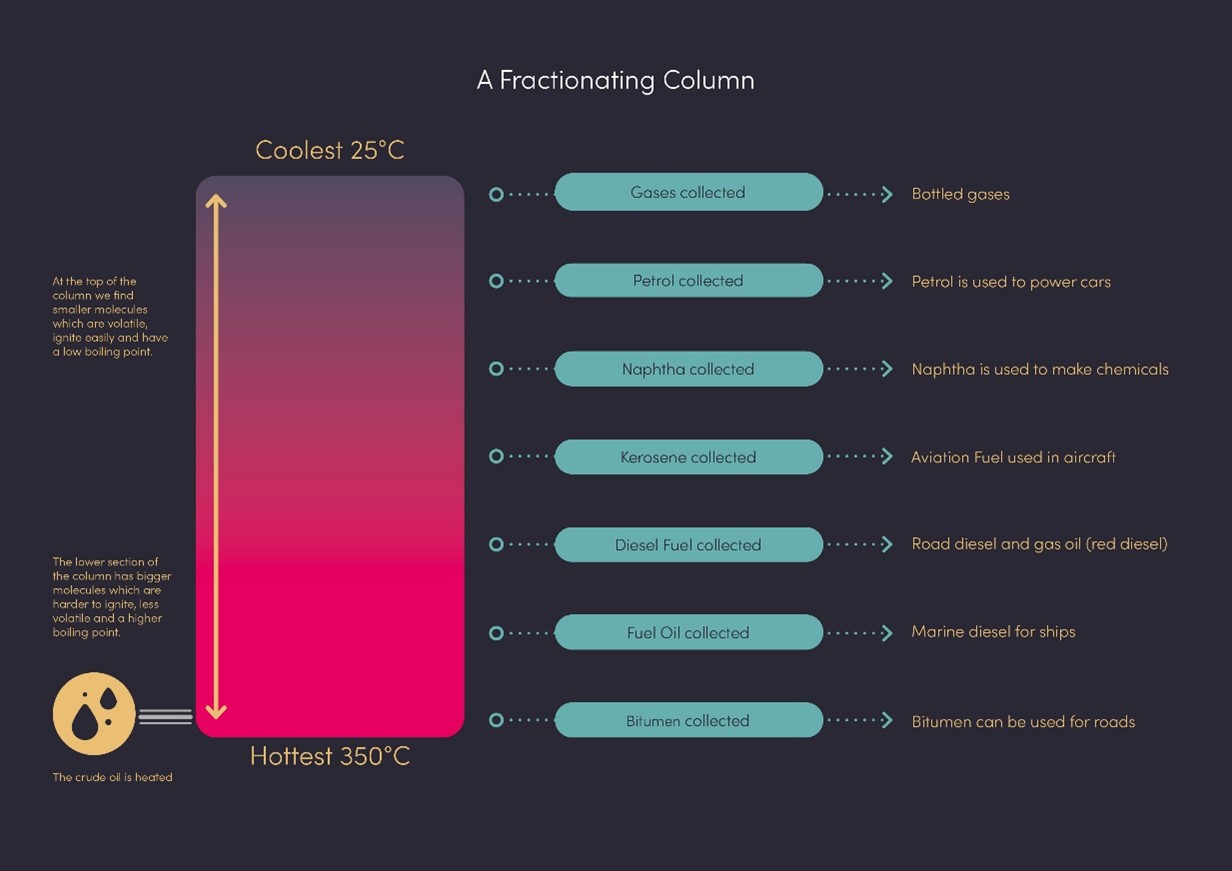
One of the first things to understand is that refineries based on fossil fuels do not only produce passenger car, marine or aviation fuels, but a bit of everything. At the start of our modern industry, the focus was not on car or marine fuels. The fuel industry was concentrated on oil lamps and aviation and was producing “wastes”: one part was too “light”, the other was too “heavy” to be used in then-conventional applications. It’s only later that these wastes became gasoline and diesel for road usage.
Secondly, it’s important to note that it’s extremely difficult to change the balance of fuel production, as output is heavily dependent on input crude. It is naïve to think you can simply suppress one route: suppressing diesel would not allow you to produce a lot more of something else, it would simply create a waste if the same quantity of crude is still converted.
Does it mean that we are doomed to produce fossil fuels forever? Not necessarily! It is up to us (businesses, engineers, politicians and also users) to optimise what we do with these fuels, decreasing our dependency on fossil crude by benefiting from a variety of options and more importantly, developing other type of fuels that are not of fossil origin. This is where renewable fuels play a crucial role.
We can be proactive and reverse the problem: instead of looking for usage for fuels, let’s focus on energy management. The end goal is to develop cleaner ways to produce energy with renewable resources (sun, wind, hydropower) and use this energy efficiently. As electrification progresses, part of this energy can be consumed through battery electric propulsion systems. Meanwhile, all existing engines (cars, trucks, planes, boats etc.) still need to be able to run in the cleanest possible way following the requirements laid out in Fig. 1. But how?
Why is developing cleaner fuels vital?
We have no choice! Most of our existing cars, trucks, planes, boats need specific fuels for propulsion. What would decarbonisation mean for them? Throwing away more than 1.4 billion cars and 25,000 planes is not the best way forward. Firstly, we do not have the capacity to dismantle and recycle all these vehicles over a short period. Secondly, this would generate billions of tons of CO2 and would take many years to compensate because of the introduction of new vehicles with new propulsion technologies (which themselves are not completely neutral regarding GHG).
As a result, operating these vehicles until their end of life might be the most ecological approach. The average age for a truck in Europe is 13 years and for a cargo ships, about 25 years. Whatever politicians and media tout, it is simply not possible to completely get rid of fuels within the next 20 years, whatever the adoption of electrification. Yet, this does not mean that we have to keep it business as usual.
Our only chance to comply with our CO2 reduction objective is to develop low carbon fuels that are compatible with existing engines.
Interestingly, gasoline and diesel fuels were not invented to fit in given engines, but rather engines were tailored to use the available wastes from refineries. It’s only with time that the previous leading applications became history and transportation fuels became predominant.
Early engines were just burning what was available, and we very rapidly concluded that some fuel properties were much more important than others. For example, as early as the 1920’s, engineers found a relationship between the fuel quality and knocking, performance and efficiency [Ricardo]. From this point on, many iterations between fuel specifications and engine technical developments were made.
Fuel Specifications
Commercial fuels do not correspond to a “unique recipe”. In fact, the exact composition of commercial fuels is unknown, not because they cannot be given but rather because this knowledge is useless.
To give you an example, the Unleaded Gasoline sold in Europe does not correspond to a unique composition, but to a set of specifications to be fulfilled. The EN228 specification lists parameters and a prescribed range of values for each: any liquid simultaneously matching all ranges is a gasoline, no matter its origin or production process.
Let’s consider evaporation as an illustrative parameter. For your engine to work properly, fuels must evaporate, i.e. transition from liquid to gas state as only gases burn. Yet, excessive volatility is not desired either because you want to control when the fuel evaporates.
If you live in Spain where summers are hot, you want fuel to evaporate just before combustion, not to evaporate from your fuel tank. There is consequently an upper evaporation limit, restricting the type of molecules composing your fuel. On the other hand, during winter in Germany, it’s difficult for fuel to evaporate (evaporation rate depends on temperature), so a lower evaporation limit is imposed to ensure that cars can start.
Local climates are so different between summertime in Southern Europe and wintertime in Northern Europe, that a single range of evaporation limits would not be practical. This explains why EN228 gives different ranges for each property depending on location and season. In any country, winter fuel is different from summer fuel for production and to ensure it is fit for purpose.
From this, it’s easy to see why a fuel is not defined by a composition but rather by the properties it complies with. This is true whatever the fuel type (on-road, marine, aviation) and the standards (European, Chinese, American etc.)
Fuel Properties
Existing cars, trucks, boats and aircrafts have been developed towards a common understanding of the physical and chemical properties of fuels defined by specifications. It is important that fuels and their usage don’t damage related engines.
Here are some examples of properties required by a fuel:
- Cleanliness – it characterises the propensity of a fuel to make deposits and “sludge” (sort of muddy aspect). These deposits can build up within the engines and systems themselves (injectors, valves, pistons, etc.) but this can also be related to storage (sediments in tank) or degradation when ageing.
- Combustion – this includes lots of properties such as the resistance / propensity to auto-ignite. Depending on the type of application (gasoline or diesel), you are looking for resistance to auto-ignition (knocking in gasoline) or ease of autoignition (diesel). Depending on the fuels, these properties are named “Octane Number” (for gasoline) or “Cetane Number” (for diesel). Combustion properties can also be related to the heat of combustion or to the flame speed.
- Volatility – fuel needs to evaporate sufficiently for cold starting but also not excessively for safety and pollution reasons. A cloud of fuel pollutes the environment and is a hazard in enclosed spaces.
- Safe storage and usage – this includes, for example, the “Cold Filter Plugging Point” (temperature at which crystals form on hydrocarbons when you reduce the temperature), the “flash point” (the lowest temperature at which an external ignition could ignite the vapour of a stored fuel) and the “conductivity” (whether a charge of static electricity will build up during pumping operations).
- Compatibility with engines parts such as injectors, pumps, etc. – if the fuel is corrosive, it is destroying its environment (tank, pump, pipes, engine). If it is too thick (high viscosity) and has a low density or a high surface tension, it would prevent the injectors introducing the right amount of fuel.
- Toxicity and health – some compounds are such health hazards that they are banned or limited in quantity within commercial products (benzene, aromatics, etc.)
Despite a strict set of rules and property range, the exact composition of a commercial fuel is somehow flexible inside a formulation window. These properties are not linked to any raw material and none require fuel to be derived from crude oil. In this case, why not achieve these properties using sustainable fuels?
Sustainable fuels
Fuels can be “manufactured” from various raw materials and are deemed sustainable when not originating directly either from crude oil or from plants. Contrary to 1st Generation bio-fuels, such as 1G ethanol and FAMEs (not UCOME) which rely on agriculture (wheat, rape, soy, palm, sugar etc.), sustainable fuels are not in competition with the food supply.
Instead these fuel types are classed as 2nd generation or better, “sustainable” fuels. They use sources of energy that would otherwise be lost in the form of waste (e.g. wheat chaff), or at least not easily stored if not converted into a synthetic liquid. We also group “e-fuels” into our sustainability criteria, utilising renewable electricity and carbon recovery to produce a synthetic liquid, that much like advanced biofuels, can be used like a traditional fossil fuel. These types of fuels are not new but were historically too complex and expensive to produce on scale, meaning there was little attention paid to the potential GHG savings (80%) they provide.
The idea behind sustainable fuels presents a lot of advantages such as:
- They can be made from waste raw material such as CO2 capture, by-products from other industries, landfill waste, industrial waste, agricultural waste etc.
- They can be used as an energy storage method when electricity production exceeds demand (renewable electricity production does not follow demand, so excess production might occur and storage avoids wasting).
- They can be compatible with existing engines and can be used alone or in blend with fossil fuels and bio-fuels
- They do not need any infrastructure development for distribution
- They would reduce CO2 emissions of any existing engines and vehicles now, saving mining and manufacturing CO2 emissions.
- An almost infinite number of molecules designs available.
There is still a lot of room to help in the development of sustainable fuels in their many and varied guises, like the readily available 2nd Generation biofuels, all the way through to the burgeoning technologies of “e-fuels”.
If you want to know more about sustainable fuels and their application in motorsport, agriculture, aviation, marine, etc. contact us today to benefit from our decades of expertise in fuel chemistry and application knowledge.

